Abstract
Introduction: Allogeneic stem cell transplant (allo-SCT) and checkpoint blockade both have efficacy in some lymphoid malignancies. However, the safety of allo-SCT after programmed cell death 1 (PD-1) blockade in these patients (pts) remains a question of high clinical interest. We present data from an analysis from 4 KEYNOTE phase 1-3 studies to assess the incidence and severity of complications in pts who received allo-SCT following pembrolizumab therapy.
Methods: The analysis included pts with a known transplant date who received an allo-SCT within 2 years of the last dose of study pembrolizumab from the KEYNOTE-013 (NCT01953692, n=20), KEYNOTE-087 (NCT02453594, n=31), KEYNOTE-170 (NCT02576990, n=5), and KEYNOTE-204 (NCT02684292, n=14) trials. Descriptive statistics were used for prespecified complications of interest following allo-SCT. The cumulative incidence of acute grade 2-4 graft versus host disease (GVHD), acute grade 3-4 GVHD, and chronic GVHD was estimated. The corresponding competing risk events were death without acute grade 2-4 GVHD, death without acute grade 3-4 GVHD, and death without chronic GVHD, respectively. In addition, the cumulative incidence of transplant related mortality (TRM) post-allo-SCT was estimated with relapse as a competing risk. The Kaplan-Meier method was used for estimation of progression-free survival (PFS) and overall survival (OS).
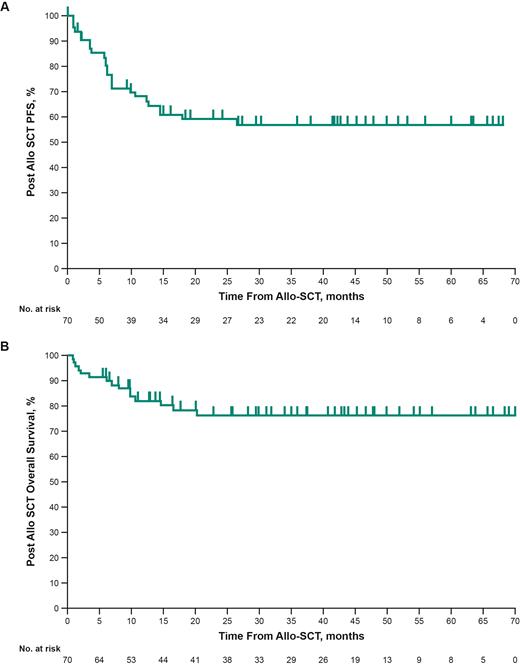
Results: Seventy patients were evaluable for this analysis. Median age was 30 y (range, 18-65), 57 (81.4%) had classical Hodgkin lymphoma, and the rest had non-Hodgkin lymphoma. Sixty-nine pts (98.6%) received pembrolizumab monotherapy and the remainder received pembrolizumab in combination with lenalidomide. Median duration on study treatment was 5.34 months (range, 0.72-29.60) and median time from last pembrolizumab dose to first allo-SCT was 4.6 months (range, 1-20). Post-pembrolizumab and before allo-SCT, 49 pts (70.0%) had intervening anticancer regimen; 34 (48.6%) had active disease, 31 pts (44.3%) were in remission at time of transplant, and 5 pts (7.1%) had unknown disease status. Forty-four pts (62.9%) received reduced-intensity conditioning, 25 pts (35.7%) received full-intensity conditioning, and 1 pt (1.4%) had missing data. In terms of donor source, 31.4% were haploidentical, 25.7% matched sibling, 31.4% matched unrelated. A total of 55 pts (78.6%) developed GVHD, 54.3% with acute GVHD and 24.3% with chronic GVHD. The estimated cumulative incidence was 0.41 (95% CI, 0.30-0.53) for grade 2-4 acute GVHD and 0.20 (95% CI, 0.12-0.30) for grade 3-4 acute GVHD, both at 6 months post-allo-SCT and was 0.21 (95% CI, 0.12-0.31) for chronic GVHD at 1 year post-allo-SCT. Common sites affected by chronic GVHD were skin, liver, and oral mucosa. Other predetermined complications, including critical illness, immune-mediated adverse events, pulmonary complications, and venoocclusive liver disease, occurred in 32 pts (45.7%). With a median follow-up, defined as the time from allo-SCT to data cutoff or death, of 30.9 months (range, 0.85-71.2) , the post-allo-SCT median PFS was not reached (NR) (95% CI, 14.5-NR) and the 30-month post-allo-SCT PFS rate was 56.8% (95% CI, 42.9-68.5) (Figure A); median overall survival (OS) was NR (95% CI, NR-NR) and the OS rate at 12 months was 82.2% (Figure B). The estimated cumulative incidence for TRM at 18-month post-allo-SCT was 0.11 (95% CI, 0.05-0.21).
Conclusions: The incidence of acute and chronic GVHD in this population seems similar to that from historical data (40%-72% and 30%-70%, respectively), although the incidence of severe acute GVHD may be higher than a typical modern allo-SCT series. Despite this, the TRM was low. Furthermore, PFS in the subgroup of pts with cHL was favorable compared to historical series of pts without prior PD-1 blockade, which is consistent with other studies in this population. Altogether, this analysis provides reassurance that allo-SCT is feasible for pts after PD-1 blockade, with PFS and OS outcomes that may in fact be better than historical benchmarks.
Armand: Merck: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy; Affimed: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Infinity: Consultancy; ADC Therapeutics: Consultancy; Celgene: Consultancy; Morphosys: Consultancy; Daiichi Sankyo: Consultancy; Miltenyi: Consultancy; Tessa: Consultancy; GenMab: Consultancy; C4: Consultancy; Enterome: Consultancy; Regeneron: Consultancy; Epizyme: Consultancy; AstraZeneca: Consultancy; Genentech: Consultancy, Research Funding; Roche: Research Funding; Tensha: Research Funding; Otsuka: Research Funding; Sigma Tau: Research Funding; IGM: Research Funding; Kite: Research Funding. Kuruvilla: Incyte: Honoraria; Gilead: Honoraria; Novartis: Honoraria; AbbVie: Honoraria; TG Therapeutics: Honoraria; Seattle Genetics: Honoraria; Roche: Honoraria, Research Funding; BMS: Honoraria; Amgen: Honoraria; Pfizer: Honoraria; Janssen: Honoraria, Research Funding; Merck: Honoraria; Karyopharm: Honoraria, Other: Data and Safety Monitoring Board; AstraZeneca: Honoraria, Research Funding; Antengene: Honoraria; Medison Ventures: Honoraria. Herrera: ADC Therapeutics: Consultancy, Research Funding; Kite, a Gilead Company: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Gilead Sciences: Research Funding; Seagen: Consultancy, Research Funding; Tubulis: Consultancy; Takeda: Consultancy; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Karyopharm: Consultancy. Ribrag: Argen-X: Research Funding; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; MSD Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Epizyme: Honoraria, Research Funding; GSK: Research Funding; PharmaMar: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Nanostring: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals: Research Funding; Infinity Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees. Brice: Takeda: Research Funding; Amgen: Other: Travel/accommodations/expenses; Roche: Other: Travel/accommodations/expenses; MSD: Research Funding. Thieblemont: Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses , Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Hospira: Research Funding; Bayer: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses . Von Tresckow: Pentixafarm: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other: Congress and travel support, Research Funding; Novartis: Consultancy, Honoraria, Other: congress and travel support, Research Funding; AstraZeneca: Honoraria, Other: congress and travel support; Amgen: Consultancy, Honoraria; AbbVie: Other: congress and travel support; Kite-Gilead: Consultancy, Honoraria; BMS-Celgene: Consultancy, Honoraria, Other: congress and travel support; MSD: Consultancy, Honoraria, Other: congress and travel support, Research Funding. Kim: Merck: Current Employment, Other: Current Stockholder. Orlowski: Merck & Co., Inc.: Current Employment, Other: Current Stockholder. Chakraborty: Merck & Co., Inc.: Current Employment, Other: Current Stockholder. Marinello: Merck & Co., Inc.: Current Employment, Other: Current Stockholder. Zinzani: Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck Sharp & Dohme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmuneDesign: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Portola: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal